If you haven’t read it yet, you can check out my post here for a brief introduction on the importance of having FDA licenses such as a License to Operate (LTO) and a Certificate of Product Registration (CPR).
In this post, I’ll be taking you to a deep dive on how to get an FDA LTO, what needs to be prepared and what FDA issuances (think memos) are relevant. There are many different types of LTO but we’ll be focusing more on getting a License to Operate as a Food Manufacturer.
LTO is a secondary license that allows a particular company to do business in food. Apart from basic documents, FDA requires food manufacturers, traders, re-packers and distributors to get an LTO before doing business with the public. Food products are considered health products. Therefore, having an FDA LTO is one way of assuring the public that the activities of food companies do not harm them. FDA does this by inspecting each and every food manufacturer that tries to apply for an LTO then evaluates their food safety system in accordance with current guidelines.
Some quick notes as you are starting with your FDA journey: It’s better to have a qualified person such as an experienced food technologist (for food) or a licensed pharmacist (for drugs) managing your document filings. If you get the correct professionals at the onset, they will make the application process more efficient. However, if you still don’t have the resources to hire extra help, just make sure to read and understand all the necessary FDA guidelines to properly handle the application.
Moreover, have the following documents ready and create PDF or PNG copies (should be clear and less than 2 MB file size). You will need these during the application process.
- Notarized Authorization Letter (details of which are in step 1)
- Proof of Business Name Registration – this can be your SEC / DTI / CDA certificate or your current Business or Barangay Permit.
- Proof of Income – this can be your Latest Audited Financial Statement or Income Tax Return for the current year. For a new business without this, you can write a letter or promissory note indicating that you have yet to get such documents.
- Government-issued valid IDs of the authorized representative and company owner. No need to make digital copies of these. Just have them ready during the application process.

Steps in Getting a License to Operate (Food)
- Step 1: GET AN FDA E-PORTAL ACCOUNT – FDA related licenses are filed using their e-portal system. You can access it here. But before you can use it, you need to have an e-portal account. Unlike creating an account in Gmail or Facebook, creating an e-portal account takes a little bit more effort.
- Prepare a notarized copy of an authorization letter following FDA’s format. Authorized representatives should be employed by the company. They can come from departments such as quality assurance, research and development, production management or from senior management. For smaller businesses that do not yet have technical personnel, the owner can be the authorized representative.
- E-mail the notarized authorization letter as a pdf attachment to fdac@fda.gov.ph and pair@fda.gov.ph. The e-mail should follow the prescribed format. In writing the e-mail, the subject field should contain “Request for e-LTO User Account.” The body of the email should have the following information:
- E-mail address of representative
- Name of representative
- Position in the company of representative
- Contact number (company or representative)
- Company name
- After one to three days, you should get a reply from FDA indicating your e-portal username and password. You can now use this to log-in to the FDA e-portal system.



- Step 2: LOG-IN TO THE FDA E-PORTAL SYSTEM – visit eportal.fda.gov.ph or click the link here. Click on New Case -> License to Operate (Data Capture) -> Start Case. This should bring you to the the “Declaration and Undertaking” section. Read this and scroll to the bottom to agree. After agreeing and clicking continue, follow along each and every window of the application process. Download the order of payment and pay to any authorized payment channels. Some points to remember:
- Make sure to input the company name and address as it appears on your business documents. It would be time consuming to have this changed after the LTO has been released.
- Upload the needed documents in PDF or PNG format. The e-portal system only accepts these formats. Ensure also that the file size does not exceed 2 MB. There have been cases of applications being delayed because the uploaded documents were too large or illegible.
- After downloading the order of payment, don’t forget to click “Next” or “Continue”. It should show you the “Assign Task” page. This is critical because in this page, you have to click “Continue” again for your application to properly proceed. There are applicants who often forget to click this very last page leaving their applications not submitted. To know that you’ve properly done this, your application should disappear from the “Draft” folder and appear in the “Participated” folder.
- Before the pandemic, application payments can be made over-the-counter, via FDA’s cashier services located at the Food and Drug Action Center (FDAC), Starmall, Alabang, Muntinlupa City. However, because of COVID-19 restrictions, payments are deposited to FDA’s Land Bank of the Philippines’ bank account. Always check the FDA website for any announcement on changes of their payment channels.


- Step 3: WAIT FOR YOUR AUDIT SCHEDULE AND PREPARE FOR INSPECTION – After successfully paying the application fees, it generally takes a few weeks (or months! depending on how busy FDA gets) before your assigned inspector contacts you to schedule an audit. An audit is a pre-requisite needed to be passed before you get your LTO. Audits ensure that the applicant company complies with current food safety guidelines endorsed by the FDA. These guidelines are called GMP or Good Manufacturing Practices. There are a lot of things to consider in preparing for an audit. It is best to consult an experienced food technologist on what needs to be done and what documents to be prepared. Even without the help of a food tech yet, you can prepare the following basic documents the inspector might look for during the visit:
- Health IDs or Medical Certificate of all food handlers
- Microbiological test report of the company’s food products
- List of suppliers with addresses and contact details
- Sample labels and packaging
- Basic business documents and a copy of your application fee receipt or deposit slip

- Step 4: WAIT FOR THE AUDIT RESULTS AND CORRECT ANY DEFICIENCIES – It’s rare to pass the audit during the first inspection. Usually, there are a few issues that the applicant company need corrected in order to satisfy the comments of the auditor. After the audit, check the e-portal regularly for any non-compliance you need to address. Your application should appear in the “Inbox” folder. Click on it until you arrive at the compliance page. This page shows what you need to submit. You should provide any documents or pictures your inspector asks because your application won’t proceed if these issues go uncorrected. After submission, visit the e-portal from time to time to check on the status of your application. Some applicants may need to go back and forth with their inspectors during the compliance phase. To avoid this, you need to pay attention to the details of any document submissions as incorrect submissions will definitely delay the application process.
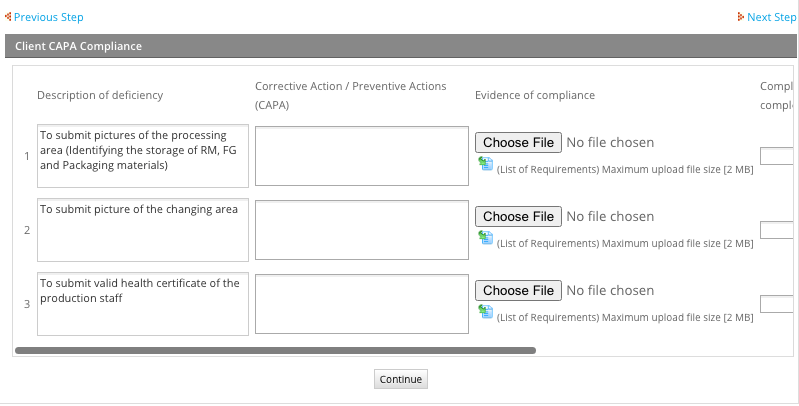
- Step 5: AFTER COMPLIANCE, LTO GETS RELEASED – When all deficiencies are corrected and all lacking documents submitted, your LTO application gets processed for released. Note that before getting your LTO, it passes through two different departments under FDA. Your application undergoes an approval process within the Regional Field Office (RFO). This office is where your inspector is based. After passing through RFO, your application is forwarded to the Center for Food Regulation and Research (CFRR) for final evaluation. CFRR is sort of a clearing house for all LTO applications related to food. They evaluate the document completeness and compliance of an applicant company to related FDA rules or issuances. Apart from LTO applications, CFRR also processes Certificate of Product Registration (CPR) applications. There are occurrences where CFRR tags LTO applications as deficient. Always check your e-portal account for any other documents they need from you in order to complete your application. Addressing any non-compliance is similar to Step 4. When all is clear with CFRR, a copy of your approved LTO is forwarded to your e-portal account. You can then just open it, download and print. Before this improvement by the FDA, all copies of approved LTOs are either picked-up at the FDAC or snail-mailed to the applicant company.
Final Thoughts

Not going to lie, the LTO application process is long and tiresome. It’s very challenging for even an experienced professional to handle. But all these steps are necessary in order to ensure that all food products that get released into the market are safe for consumption.
Need professional help? Click this link.
You can download copies of relevant FDA issuances related to the LTO application process here:

Hi. Thank you for this article. It is really a big help to those who wants to apply for an LTO. I have a question tho in regards with our LTO.
Our LTO activity was Food distrubutor/importer and it expired March 30, 2021. We want to change our LTO activity to Food distributor/wholesaler.
What type of LTO application should we get? Should it be initial since we are going to filed it after 120th day of our LTO expiration?
Thank you.
LikeLike
I want to packages and distribute a drug-free food supliment. We won’t manufacturer anything only mix together existing food supliments and market under our name. What LTO do I need before we apply for CPR? Thanks
LikeLike